Science & Technology
Developing a Treatment for ASD
Despite the high prevalence of Autism Spectrum Disorder (ASD), there are no FDA-approved medications for the core symptoms. Furthermore, many of the medications used to treat secondary symptoms of ASD come with significant side effects.1There is emerging evidence that abnormal Nitric Oxide signaling, and the subsequent cellular stress are linked to ASD and may drive the associated symptoms. 2-6Therefore, we aim to target Nitric Oxide signaling in the brain to develop a novel treatment for the core symptoms of ASD.
The Role of Nitric Oxide
Nitric Oxide (NO) is a neurotransmitter and key signaling molecule in the brain that modulates physiological neuronal and synaptic functions.7,8However, aberrant signaling and high levels of Nitric Oxide can result in nitrosative stress, which leads to toxicity and disrupts the normal functioning of neurons in the brain leading to autism-like behavioral deficits.6Scientific studies have shown elevated nitrosative stress in both animal models of Autism Spectrum Disorder (ASD) and human patients.5,6,9

Targeting NO Production in the Brain
Neuronal nitric oxide synthase (nNOS) is the enzyme responsible for Nitric Oxide production in the brain. As nNOS is found in neurons, selectively targeting this protein provides an opportunity to regulate Nitric Oxide production within the brain.
Our Hypothesis: Mechanism of Action
High Levels of Nitric Oxide Lead to Cellular Dysfunction in Brain
 NOS overactivation produces high levels of Nitric Oxide, leading to increased levels of reactive nitrogen and nitrosative stress. The result is cellular toxicity and neuronal dysfunction.
NOS overactivation produces high levels of Nitric Oxide, leading to increased levels of reactive nitrogen and nitrosative stress. The result is cellular toxicity and neuronal dysfunction.
Inhibiting nNOS to Treat ASD-Like Phenotypes
 Inhibition of nNOS activity lowers Nitric Oxide levels and reduces nitrosative stress, rescuing neuronal abnormalities.
Inhibition of nNOS activity lowers Nitric Oxide levels and reduces nitrosative stress, rescuing neuronal abnormalities.
Our Results
Our preclinical work shows that inhibiting nNOS activity reduces nitrosative stress in the brain and reverses ASD-like phenotypes in multiple mouse and cellular models of ASD. We proved this in patients’ stem cells.6
Lowered Nitrosative Stress
Inhibition of nNOS reduced nitrosative stress and signs of aberrant NO signaling in the brain. Lowering reactive nitrogen species through nNOS inhibition can result in healthy cellular conditions.

Increased Number of Dendritic Spines
Dendritic spines are a structure in neurons that play a critical role in synaptic transmission and have been shown to be reduced in ASD patients. nNOS inhibition results in an increased number of dendritic spines in the brain.

Reduced Anxiety-Like Behavior
nNOS inhibition reversed anxiety-like behavior when tested during an elevated plus maze experiment. In this test, treated mice spent more time exposed in the open arms of an elevated structured as opposed to that of untreated mice.

Increased Novel Object Recognition and Interest
nNOS inhibition increased the interest in novel objects over familiar one, suggesting an improvement in object memory and exploratory behaviors.

Increased Sociability
Inhibition of nNOS resulted in increased sociability and overall interaction with other mice in several preclinical models of ASD.

nNOS Inhibition Reverses ASD-Like Behavior in Three Different ASD mouse models
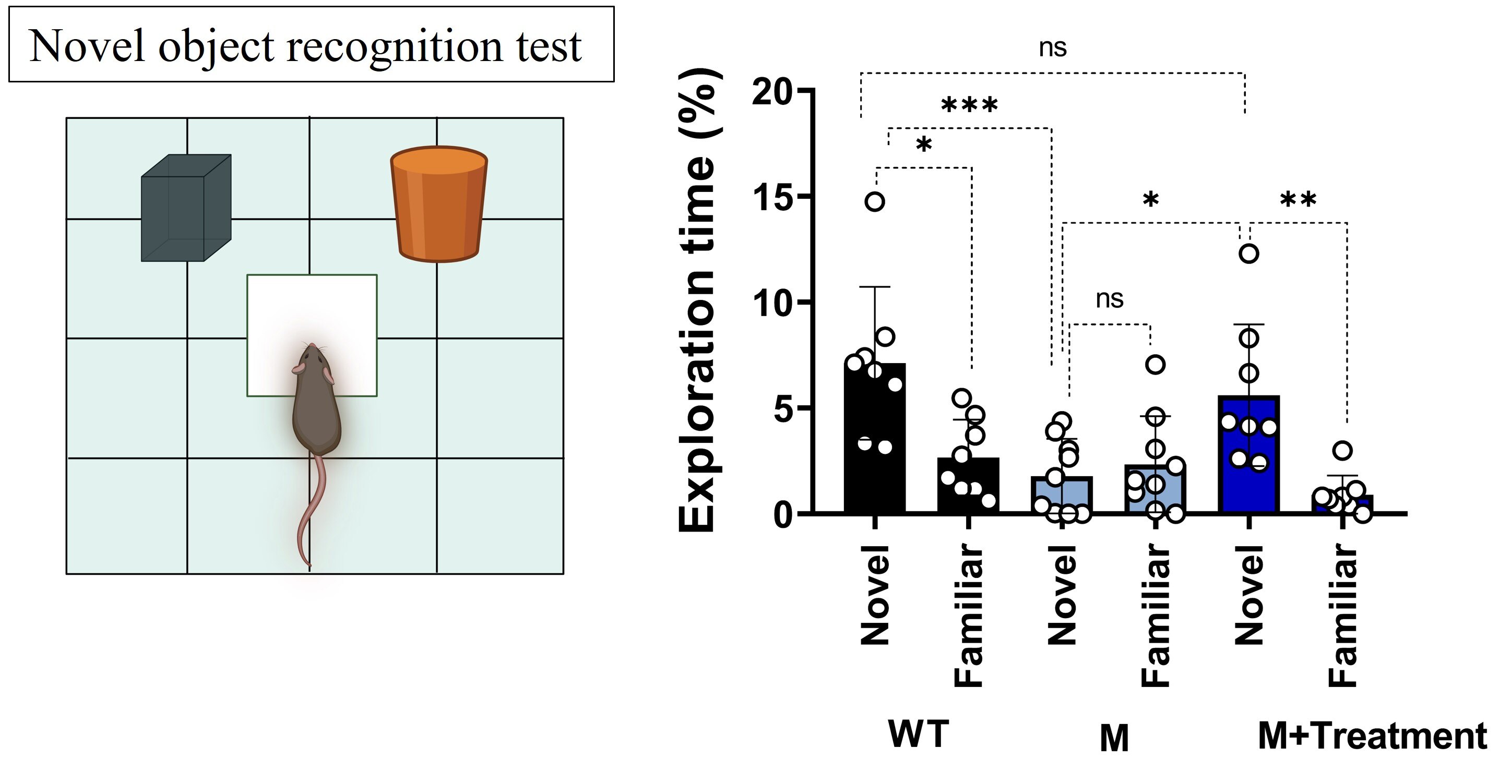
Increased Novelty-Seeking and Reduced Restricted Behavior

Increased Sociability

Reduced Anxiety-Like Behavior
WT (wild type mice), M (Shank3 mutant mice), M+Treatment (Shank3 mutant mice treated with an nNOS inhibitor). Data is presented as mean ± SD. Statistical significance was determined using a two-way ANOVA with Bonferroni’s multiple comparisons tests. *p<0.05, **p<0.001, ***p<0.0001, and ns=non-significant.
Our Timeline

* Our lead compound BA102 is not the same one used in the publication. Bridging studies proving similar efficacy have been completed. *
References
1. Shurtz L, Schwartz C, DiStefano C, et al. Concomitant medication use in children with autism spectrum disorder: Data from the Autism Biomarkers Consortium for Clinical Trials. Autism. 2023;27(4):952-966. doi:10.1177/13623613221121425; 2. Tripathi MK, Kartawy M, Amal H. The role of nitric oxide in brain disorders: Autism spectrum disorder and other psychiatric, neurological, and neurodegenerative disorders. Redox Biol. 2020;34:101567. doi:10.1016/j.redox.2020.101567; 3. Amal H, Barak B, Bhat V, et al. Shank3 mutation in a mouse model of autism leads to changes in the S-nitroso-proteome and affects key proteins involved in vesicle release and synaptic function. Mol Psychiatry. 2020;25(8):1835-1848. doi:10.1038/s41380-018-0113-6; 4. Kartawy M, Khaliulin I, Amal H. Systems Biology Reveals S-Nitrosylation-Dependent Regulation of Mitochondrial Functions in Mice with Shank3 Mutation Associated with Autism Spectrum Disorder. Brain Sci. 2021;11(6):677. Published 2021 May 21. doi:10.3390/brainsci11060677; 5. Hamoudi W, Tripathi MK, Ojha SK, Amal H. A cross-talk between nitric oxide and the glutamatergic system in a Shank3 mouse model of autism. Free Radic Biol Med. 2022;188:83-91. doi:10.1016/j.freeradbiomed.2022.06.007; 6. Tripathi MK, Ojha SK, Kartawy M, et al. The NO Answer for Autism Spectrum Disorder. Adv Sci (Weinh). 2023;10(22):e2205783. doi:10.1002/advs.202205783; 7. Hardingham N, Dachtler J, Fox K. The role of nitric oxide in pre-synaptic plasticity and homeostasis. Front Cell Neurosci. 2013;7:190. Published 2013 Oct 31. doi:10.3389/fncel.2013.00190; 8. Sobrevia L, Ooi L, Ryan S, Steinert JR. Nitric Oxide: A Regulator of Cellular Function in Health and Disease. Oxid Med Cell Longev. 2016;2016:9782346. doi:10.1155/2016/9782346; 9. Melnyk S, Fuchs GJ, Schulz E, Lopez M, Kahler SG, Fussell JJ, Bellando J, Pavliv O, Rose S, Seidel L, Gaylor DW, James SJ. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J Autism Dev Disord. 2012 Mar;42(3):367-77. doi: 10.1007/s10803-011-1260-7. PMID: 21519954; PMCID: PMC3342663; 10. Tropea MR, Gulisano W, Vacanti V, Arancio O, Puzzo D, Palmeri A. Nitric oxide/cGMP/CREB pathway and amyloid-beta crosstalk: From physiology to Alzheimer's disease. Free Radic Biol Med. 2022;193(Pt 2):657-668. doi:10.1016/j.freeradbiomed.2022.11.022; 11. O'Gallagher K, Rosentreter RE, Elaine Soriano J, et al. The Effect of a Neuronal Nitric Oxide Synthase Inhibitor on Neurovascular Regulation in Humans. Circ Res. 2022;131(12):952-961. doi:10.1161/CIRCRESAHA.122.321631
